Science just took another big step, creating a 3D printed reproductive organ that could potentially lead to a cure for infertility in women. “A true ovarian bioprosthetic”, as explained by Teresa K. Woodruff PH.D., one of the researchers on this project. Their goal with this experimental gelatin ovary is to restore fertility and endocrine health in women who have suffered the negative side effects of cancer treatment.
Researchers from Northwesten University Feinburg School of Medicine and McCormick School of Engineering collaborated to create a precise geometric design for this surrogate ovary. The team compares this to a scaffolding structure on a building under construction, it is only designed to be there until the structure can support itself.
This evolutionary design could eventually be used for other soft organ or soft tissue applications, according to Ramille Shah “we will be able to print any architecture that would be optimal for different cell types” So maybe the future will have less of a need for live organ transplants.
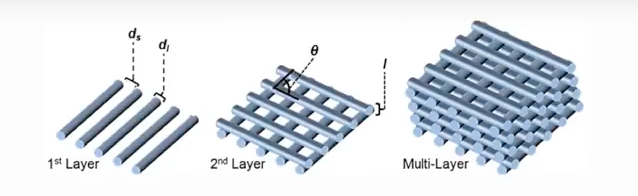
The 3D printer used to make the scaffolding structures lines the filament “like Lincoln logs” said Alexandra Rutz, co-lead author of the study and a former biomedical engineering graduate fellow in Shah’s Tissue Engineering and Additive Manufacturing (TEAM) lab at the Simpson Querrey Institute. The engineering team are able to control angles and distance between the filament to create different sized pores to fit specific needs.
This complex bioprosthetic isn’t 3D printed with normal plastic filament, the researchers used gelatin. The research paper states:
“Gelatin was selected because it is derived from collagen, an extracellular matrix protein abundant in both human and mouse ovaries, it is degradable to allow for cellular remodelling, contains cell adhesion sites and has soft, yet durable mechanical properties.”
The gelatin’s likeness to collagen prevents the body from treating the implant as something foreign, which made it a perfect home for the mice eggs, and possibly one day human eggs. See below an egg perfectly attached between the angles in the scaffolding.
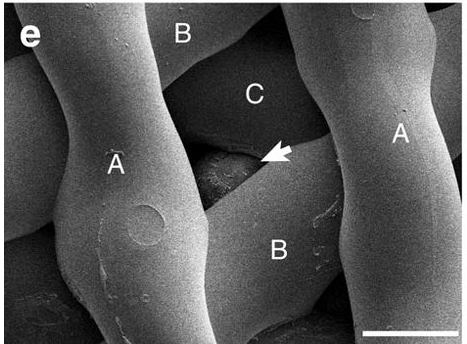
In the experiment the mother mouse’s ovaries were completely removed, and one was replaced with a 3D printed bioprosthetic. They then inserted eggs and watched as they grew into mouse pups, reaching their goal of curing infertility in mice.
Aside from the goal of allowing an infertile mother to give birth, they set forth to catalyze the release of important hormones. Monica Laronda, co-lead author of this research and a former post-doctoral fellow in the Woodruff lab said in an interview:
“What happens with some of our cancer patients is that their ovaries don’t function at a high enough level and they need to use hormone replacement therapies in order to trigger puberty,”
“The purpose of this scaffold is to recapitulate how an ovary would function. We’re thinking big picture, meaning every stage of the girl’s life, so puberty through adulthood to a natural menopause.”
The success of this goal is exciting, the mother of the mice pups was even able to produce milk for her offspring. Pores in the scaffolding structure allow room for the egg cells to mature and ovulate, as well as blood vessels to form within the implant enabling the hormones to circulate within the mouse bloodstream and trigger lactation after giving birth.
Pictures and information from this video and the full scientific report here.

 Get your own 3D Printer here on Amazon!
Get your own 3D Printer here on Amazon!


